To most effectively harness the potential impact of complex small molecules for both science and medicine, it is critical to maximize the simplicity, efficiency, and flexibility with which these types of compounds can be synthesized in the laboratory. In this regard, modern peptide synthesis, involving the iterative coupling of bifunctional amino acids represents a valuable benchmark. Amino acid building blocks are now commercially available in protected form as stable, crystalline solids, and the process of peptide synthesis is routinely automated. As a result, this powerful discovery engine is accessible to a broad range of scientists.
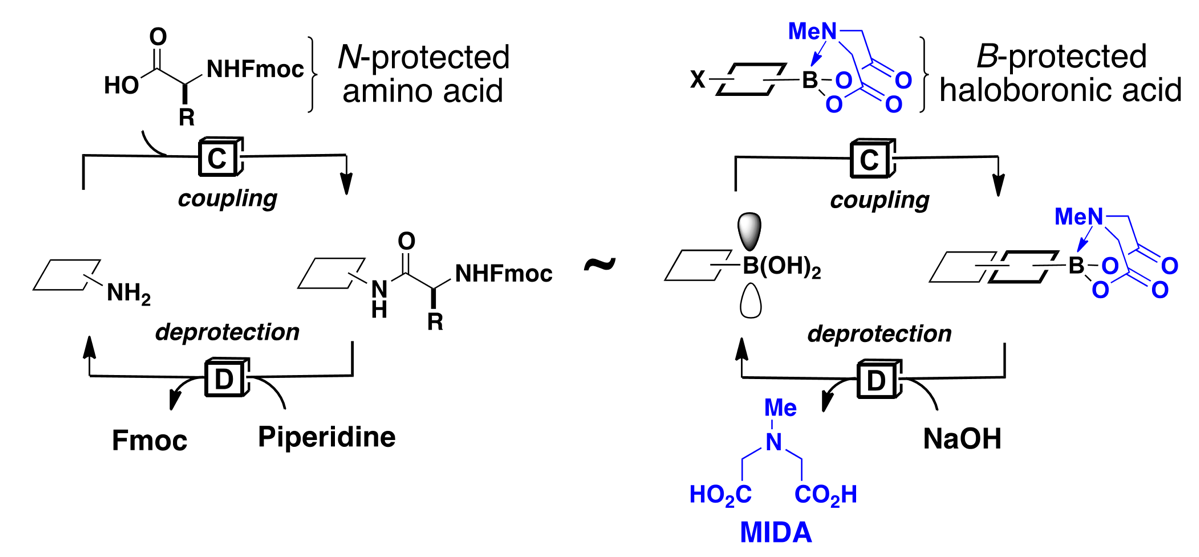
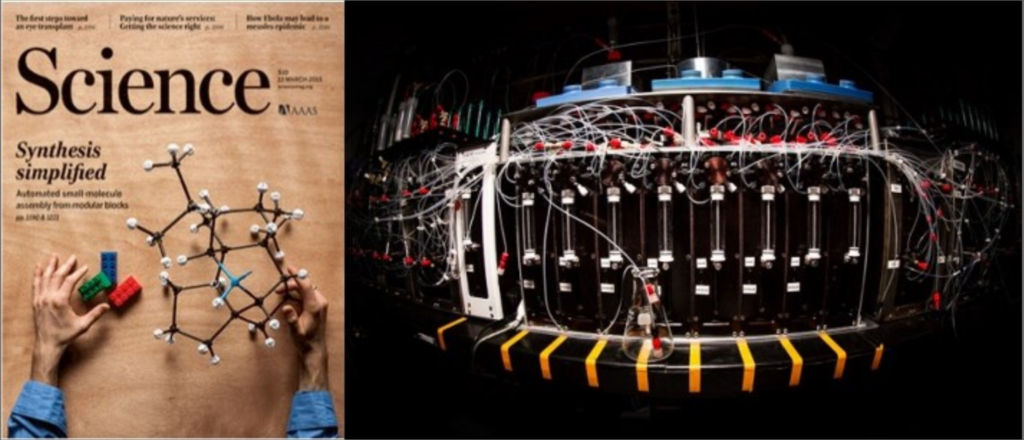
In sharp contrast, the laboratory synthesis of small molecules remains a relatively complex and non-systematized process. We have developed a simple and highly modular strategy for making small molecules which is analogous to peptide synthesis and involves iterative Suzuki-Miyaura cross-coupling of B-protected haloboronic acids (Figure 1). In this approach, building blocks are prepared (or simply purchased) having all of the required functional groups preinstalled in the correct oxidation state and with the desired stereochemical relationships. These building blocks are then brought together via the recursive application of one mild reaction. This strategy has been applied to a diverse array of natural products, drug molecules, and materials by our laboratory as well as others (Figure 2). We have even developed an automated “synthesis machine” that allows for the automated synthesis and purification of small molecules (Figure 3).
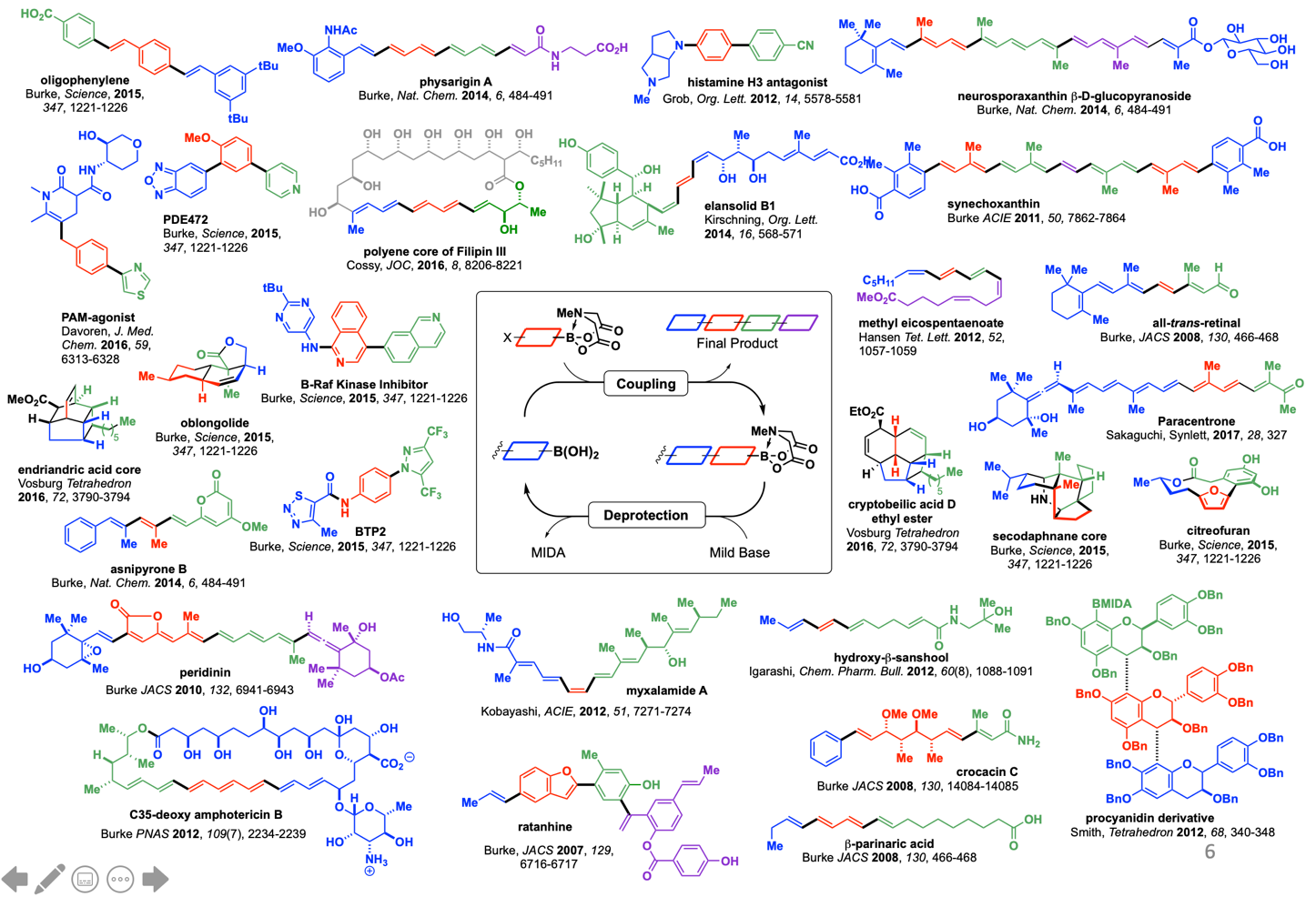
Although certain small molecules are currently more amenable to this approach than others, the rapidly expanding scope of the Suzuki-Miyaura reaction, which increasingly includes Csp2-Csp3 and Csp3-Csp3 couplings, suggests the potential for broad generality. In order to expand this strategy to synthetic targets with increased stereochemical complexity, our lab is exploring the use of Csp3 boronates and electrophiles in stereospecific Csp3 cross-coupling, and the mechanistic principles that guide the stereochemical outcome of these reactions (Figure 4).
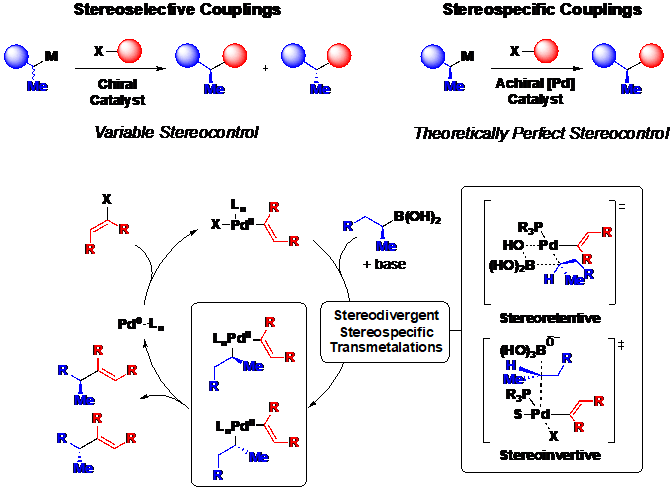
Our ongoing efforts are focused on developing Csp3 cross-coupling methods which are amenable to this iterative cross-coupling approach. Specifically, the group is using MIDA-boronate enabled modularized small molecule synthesis to develop next-generation small molecule protein mimics or “molecular prosthetics.”