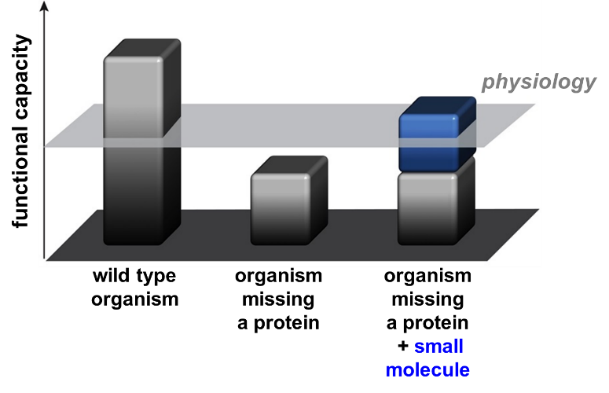
Healthy organisms are equipped with an inherent capability to function above the required level of physiology. On the other hand, organisms with missing proteins are limited in their capacity to perform normal physiological functions, rendering them in a pathological state (Figure 1). To address this issue, we hypothesized that small molecules which can perform protein-like functions to replace the missing proteins, termed as ‘molecular prosthetics’, can promote physiological restoration.
Despite the prevalence of small molecules as drugs, they are mostly utilized to modulate protein targets as activators or inhibitors. Uniquely, our lab has identified several ‘molecular prosthetic’ small molecules that are capable to replace missing proteins, such as hinokitiol and amphotericin B (AmB).
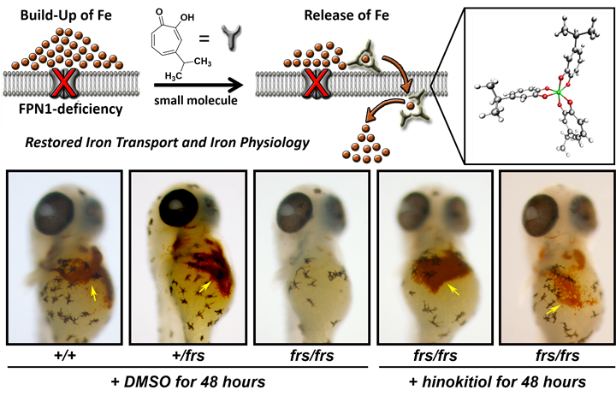
Hinokitiol is a small molecule natural product that has capability of mobilizing iron in a site- and direction-specific manner, thus restoring physiology compromised by iron dyshomeostasis caused by missing iron transporters, such as anemia (1) (Figure 2). As iron dyshomeostasis underlies various diseases, we are studying the mechanistic underpinnings of hinokitiol-mediated physiological restoration in multiple iron metabolism disorders. Furthermore, to improve the efficacy, we are also conducting structure-activity relationship (SAR) studies using derivatives of hinokitiol.
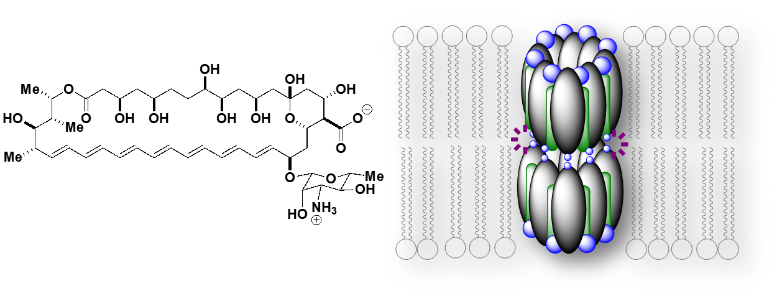
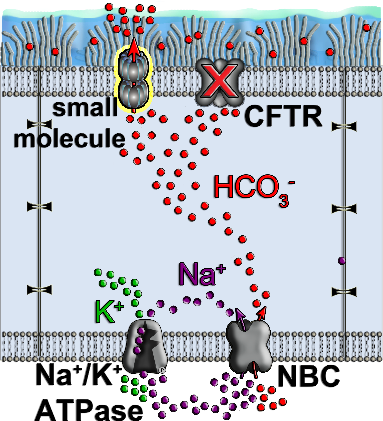
Moreover, we found that AmB is capable of transporting monovalent cations and anions through ion channel formation in the membrane. This mode of action is integral to specifically address cystic fibrosis (CF), a disease characterized by heterozygous mutations of CFTR anion channel, which compromises Cl– and HCO3– secretions (2) (Figure 3). Besides CF, there are many other channelopathies which can benefit from the unique and broad treatment option from small molecules containing the same core motif as AmB. We are now aiming to synthesize derivatives of AmB in order to tune the ion selectivity to enable a more targeted therapy for channelopathies.
On top of using small molecules as a substitute for missing transporters, we have extended the study of molecular prosthetics into ‘enzyme prosthetics’. Enzyme deficiencies underlie various metabolic disorders with limited clinical treatments. One of the diseases in particular is ornithine transcarbamylase (OTC) deficiency, a hereditary disorder characterized by excessive accumulation of ammonia in the blood due to disrupted urea cycle. OTC plays an important role in the urea cycle, especially to convert L-ornithine to citruline. We aim to synthesize and screen small molecules that are able to perform chemical reactions needed for the completion of urea cycle by combining organic chemistry, chemical biology, and biochemical approaches.
- A. S. Grillo et al., Restored iron transport by a small molecule promotes absorption and hemoglobinization in animals. Science (New York, N.Y.) 356, 608-616 (2017).
- K. A. Muraglia et al., Small-molecule ion channels increase host defences in cystic fibrosis airway epithelia. Nature 567, 405-408 (2019).